15 Answers About CO2 incl. Why It Comes Out Of The Truck Exhaust.
Burning 1 liter of diesel produces 2.65 kg of CO2.
Source: helmholtz.de
It’s not negotiable, no chance! It is a law of nature.
Does it mean you cannot influence the CO2 emission?
No, it doesn’t mean that!
In this article, you will learn a lot about carbon dioxide and how much of it is created by burning different types of fuel.
Please take a look. The article will help you to understand the topic and its connection to climate change.
What is CO2?
Carbon dioxide (the chemical formula is CO2) is a molecule of one carbon atom and two oxygen atoms.
This chemical element is an integral part of our everyday life.
It flows out of the exhausts of (almost) all trucks and cars, and we humans breathe it out ourselves.
- We breathe in oxygen-containing air,
- we eat carbonated food,
- we produce strength and CO2 in our muscles, and
- then exhale the CO2 with the air exiting our body.
- Our exhaust gas, the air we breathe, contains approx. 4% CO2. (source)
The atmosphere on Earth consists of 0.038% CO2. Because of this very small proportion, it is referred to as a “trace gas”. The same applies to some other chemical elements in the air.
For what purposes do we humans happily use CO2?
CO2 can be found in many beverages.

When CO2 forms a chemical bond with water, it is called carbonic acid.
Most of the carbon dioxide in fizzy drinks is not carbonic acid, as many people might believe, but dissolved carbon dioxide gas.
It escapes from the liquid in the form of small bubbles and creates the desired tingling effect.
At -78 °C (-108.4 °F) CO2 is frozen. It is then called dry ice. The reason for this is that it passes directly from the solid to the gaseous state when heated.
If very low temperatures are required in industrial applications, CO2 is used as a coolant. It is used, for example, in the food industry for cooling food or in mechanical engineering for the assembly of shrink fits.
At entertainment events, CO2 is used to produce fog. You may have experienced that yourself.
In medicine, medical equipment is cooled (MEG), and skin diseases are treated with frozen CO2.
CO2 is also used in fire extinguishers as an extinguishing agent
What role does CO2 play in environmental protection?
Carbon dioxide plays a very important role in the life on Earth.
In this context, the importance of CO2 is so great that scientists declare the effects of other greenhouse gases, such as methane, nitrous oxide, F-gases, etc., in carbon dioxide equivalents.
The carbon dioxide equivalent says how much stronger the greenhouse effect of these other gases is compared to the effect of CO2.
Over millions of years, a balance has developed on Earth.
But it was not always like that.
Why is CO2 in the air?
For many, many billions of years, the Earth’s atmosphere consisted only of carbon dioxide, and it was incredibly hot on Earth. There was no life.
Around 3.5 billion years ago, bacteria appeared in this hostile environment and split the CO2 into oxygen and carbon through photosynthesis.
Photosynthesis uses the energy of sunlight to break down the CO2.
Oxygen became part of the air and formed ozone, which has protected us from cosmic rays ever since.
The carbon from this process was deposited in the ground.
The conditions on earth which make life possible exist now for 350 million years. (Source: Wissenschaft-im-Dialog.de)
But we humans have been intervening in this cycle for a little more than a hundred years now. It started with the onset of the industrial age.
We take carbon (crude oil, natural gas, coal) out of the ground and convert it back into CO2. The work which results from this process is used to drive machines. The overall goal is to improve our prosperity and wealth.
By using fossil fuels, we are restoring, piece by piece, the state the earth was in before we existed.
– Not good at all! –
How is CO2 created?
In order for a CO2 molecule to be created, a carbon atom and two oxygen atoms have to exchange electrons and form a chemical bond.
During this process, heat is released.
This is called an exothermic reaction or combustion.

I will go into this in detail in many blog posts.
There is still one alternative: Combustion with a deficit of oxygen.
This creates carbon monoxide (CO).
CO is a deadly breath poison. That is not a good idea. CO2 is definitely the better choice.
In addition, CO2 is also produced in chemical processes used in industry. Processes that generate a lot of CO2 are the production of cement from limestone and the smelting of iron ore into pig iron.
Why is carbon so popular as a fuel?
In order to get the combustion process going, energy must first be supplied, the “ignition”.
After that, the combustion generates enough heat to keep the reaction going.
The combustion continues until the fuel runs out, or the temperature drops below the ignition temperature. You know that under the term “extinguishing” a fire.
Carbon is characterized by the fact that it is difficult to ignite, but after ignition delivers a lot of heat from a very small volume.
This property explains why carbon is so incredibly popular as a fuel.
Carbon can be found in different forms:
- Pure form –> Coal
- Bound to hydrogen –> Oil, natural gas
- Bound to oxygen –> Carbon monoxide and carbon dioxide
It is widely available and relatively easy to handle.
Why is CO2 produced during combustion in the engine?
The diesel engine is an internal combustion engine.
Carbon-containing diesel oil is burned inside the engine’s combustion chamber in order to generate power.
I will explain exactly how the engine works in a separate article, a precise description would lead too far at this point
Here is a short version:
So far, so good.
By the way, you can read about the connection between combustion in the engine, the power generated in the process, and energy consumption during transport in a separate article soon.
What happens when diesel is burned?
Diesel consists of several hydrocarbon molecules.
These molecules differ in the number of carbon and hydrogen atoms.
For example, Hexadecane with the formula C16H34.
One molecule of Hexadecane has 16 carbon atoms and 34 hydrogen atoms that can react with oxygen. It looks like this:
2 C16H34 + 49 O2 –> 32 CO2 + 34 H20
You can identify the two long chains of Carbon-hydrate (Hexadecane) and the Oxygen molecules from the air accompanying it.
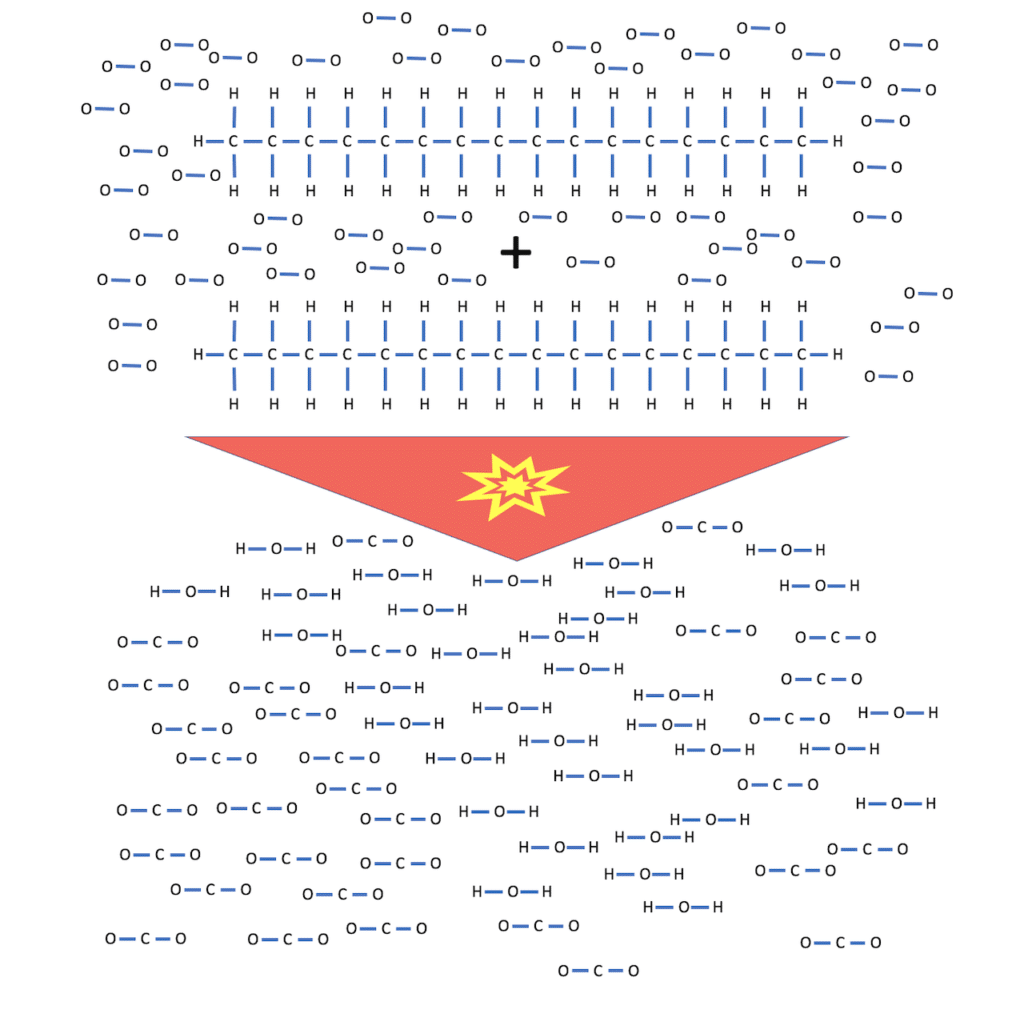
After the explosion, the long chains are broken up and only small water and carbon dioxide molecules are left over.
No matter how “clean” the combustion is, every combustion of diesel will leave CO2 behind.
The second product of the combustion reaction is water!
Really, there is a lot of water vapor coming out of truck or pass car exhausts!
Nobody talks about it because water is harmless.
I guess a lot of people don’t even know this fact.
What other combustion products are produced?
Diesel is not pure carbon hydrate, and the air is not pure oxygen.
Everything that is in the cylinder can react with the oxygen in the air as soon as the ignition has taken place. So let’s take a closer look at that.
In the cylinder, we have carbon atoms, hydrogen atoms, and nitrogen atoms, which react with oxygen during combustion and form chemical compounds.
Besides the already mentioned carbon dioxide (CO2) and water (H2O), various nitrogen oxides (NOx) are created.
Nitrogen oxides are particularly critical.
They are poisonous, acidic, and have a greenhouse effect as well.
Fortunately, there is a way to convert the nitrogen oxides back into nitrogen and water vapor with SCR catalytic converters and Adblue fluid while still in the vehicle. (Adblue is also known as “diesel emission fluid” or DEF)
Unfortunately, there is no comparably elegant way to break down CO2 back into carbon and oxygen.
Because the combustion process is extremely fast and explosive, atoms and molecules that are not completely burned also remain in the exhaust gas.
Among other things, this is soot, also known as fine dust.
Soot are particles made of pure carbon that have not found oxygen atoms to dock on.
They are so small that we breathe them up into our lungs, where they can cause health issues.
These pollutants are also defused in the truck’s exhaust system. They are burned afterward or filtered out of the exhaust gas in a soot filter.
How much CO2 is produced when diesel is burned?
How much CO2 is released can be answered quite precisely:
In the table, you find the values for diesel made from crude oil and for diesel with a blend of biodiesel, such as that used at the fuel stations in Germany.
| Diesel | Diesel with Biodiesel | |
| CO2 Tank to Wheel | 2,629 kg/l | 2,621 kg/l |
| CO2e Tank to Wheel | 2,665 kg/l | 2,657 kg/l |
| Co2e Well to Wheel | 3,174 kg/l | 3,069 kg/l |
I have extracted the following information from:
- „Berechnung von Treibhausgasemissionen in Spedition und Logistik gemäß DIN EN 16 258“ from DSLV Deutscher Speditions- und Logistikverband e.V. ,
- „CO2-Berechnung — Das Sonderheft zur Ermittlung von Treibhausgasen in der Logistik“ Verkehrsrundschau Spezial
If you want, you can read more details yourself under the two links.
The 3 values for the CO2 emissions in the table differ in scope.
The CO2 footprint for the transport of goods is based on the WTW observation scope. It is used to determine how much CO2 was caused by the transport of goods overall.
To regulate vehicle CO2 emissions, the regulators use the tank-to-wheel value. The reason is, that it is the scope of influence for the vehicle manufacturers.
Why are CO2 emissions calculated in g / tkm?
The last step to determine the CO2 emissions is still missing.
We now know the emissions per liter of diesel, and now we have to multiply by the amount of diesel used.
In freight transport, the value of CO2 emissions is related to the transported payload of one ton and a distance of one kilometer. This gives the value in g / tkm. (Grams per ton and kilometer)
In this way it is possible to compare different means of transport such as shipping, rail, road freight transport or aviation.
The absolute amount of diesel consumed is often not known.
In these cases, you can take the distance-related fuel consumption in liters per 100 km, multiply it by the CO2 value in kg or g, and divide it by the payload in tons times 100. You then get the CO2 value in kg / tkm or in g / tkm . (There is a separate article explaining the calculation of different fuel consumption types).
For this approach, the fuel consumption must be determined correctly. We will take a close look at that in my blog.
To relate the CO2 emission to the payload transported makes sense. Otherwise, the comparison would be misleading.
If I were to compare the CO2 emissions of a 40-ton truck with a 1000-ton train without taking the much larger load of the train into account, it would be unfair for the train.
In the public discussion, we encounter the CO2 emission value in two main fields:
What are the CO2 emissions of different means of transport?
I have investigated the bandwidth of CO2 emission that different carbon-based means of transport have at full load.
The data are taken out of the special edition Verkehrsrundschau (Source: Andre Kranke; So ermitteln sie den CO2 Fussabdruck, Verkehrsrundschau, 23.09.2010).
| g/tkm | CO2 TTW | CO2e TTW | CO2 WTW | CO2e WTW |
| Van | 342 | 346 | 408 | 413 |
| Truck | 32 -85 | 33 -86 | 38 -102 | 39 – 103 |
| Train | 17 – 65 | 17 – 66 | 20 – 77 | 20 – 78 |
| Barge | 8 – 53 | 8 – 54 | 10 – 63 | 10 – 64 |
| Seagoing ship | 2 – 61 | 2 – 62 | 3 – 73 | 3 – 74 |
| Cargo plane | 421 – 1712 | 427 – 1736 | 502 – 2043 | 508 – 2067 |
One can see that even with the normalization of tonne-kilometers, there are still very large fluctuations, which have different reasons depending on the means of transport.
One thing is always the case: The more cargo a means of transport can load up, the lower the relative CO2 emissions are.
The van with a loading capacity of 0.5 tons is representative of the cars. You can see how bad the balance is when fully loaded. Not even thinking about it if there is only one driver in it.
Does the combustion of natural gas or petrol produce less CO2?
Petrol and natural gas have a higher hydrogen/carbon ratio than diesel. As a consequence, more water and less carbon dioxide are produced when they are burned.
This reads well. More water and less carbon dioxide would be good for the environment.
When we look up the CO2 Values in the literature, we find the values in the table below.
| Fuel Type | CO2 Emission TTW | CO2e Emission WTW |
|---|---|---|
| Diesel with 7% Biodiesel | 2,621 kg/l | 3,069 kg/l |
| Petrol | 2,362 kg/l | 2,781 kg/l |
| Compressed natural gas (CNG) | 2,540 kg/kg | 3,229 kg/kg |
As I have introduced already, we typically look into different scopes.
The CO2 Emission Tank to Wheel (TTW) which we assign to the vehicle manufacturers.
The CO2e Well to Wheel (WTW) tells us the complete CO2 emission into the atmosphere.
At first glance, the numbers look quite similar, with diesel being the worst in the TTW column.
These numbers are often used when people talk about this matter.
But this is not really correct.
That’s why we’re delving even deeper into the subject.
CNG has a remarkable big difference between TTW and WTW.
The reason is the very low density of the gas and the resulting large volume. It causes many transport emissions on the way from the source to the filling station, as many containers, i.e. many tankers, are required. This value varies greatly, as it depends on the natural gas production site, the refinery and the type of transport (tanker vs. pipeline).
After all, the gas often comes from overseas.
For the following discussion, I put the TTW value in brackets, so that we can always see both scopes.
But let’s look a little deeper into the matter.
At first, we realized that CNG is in different units. We can convert everything into mass.
| Fuel type | CO2 Emission | Density | CO2 Emission per fuel mass |
|---|---|---|---|
| Diesel | 3,069 (2,621) kg/l | 0,83 kg/l | 3,697 (3,165) kg/kg |
| Petrol | 2,781 (2,362) kg/l | 0,74 kg/l | 3,758 (3,191) kg/kg |
| Compressed natural gas (CNG) | 3,229 (2,540) kg/kg | 3,229 (2,540) kg/kg |
Now Diesel and Petrol move closer together. Gas still seems to be better.
But this is still not the end. We need to look at it from an energy point of view. Let’s add the caloric value, which stands for the energy released during combustion.
| Fuel type | caloric value | CO2 Emission per Energy | percentage |
|---|---|---|---|
| Diesel | 11,97 kWh/kg | 0,309 (0,264) kg/kWh | 100% |
| Petrol | 12,19 kWh/kg | 0,308 (0,262) kg/kWh | 99,7(99,2)% |
| Compressed natural gas (CNG) | 13,16 kWh/kg | 0,193 (0,245) kg/kWh | 79,3(73,1)% |
We see that diesel and petrol are very close to each other. Gas shows an advantage of 20%.
Now, that is still the input side of the story. What we need is the output side, and there it becomes complicated.
The internal combustion engine needs to trade the heat from the combustion into torque and rpm.
In this process, efficiency occurs that we still have to take into account if we want to make the comparison of the fuel types correctly.
Because of the very different properties of the fuel, the engine has to deal with it in different ways. I am going to explain the diesel combustion process in a separate article.
Engineers work hard to improve all sorts of engines. It can therefore not be concluded how big the difference in efficiency and CO2 emission is, without the information of the particular fuel consumption.
According to the literature, the average Otto process is between 5 to 10% less efficient than the diesel process.
For the gasoline engine, this means additional consumption and emissions compared to diesel.
It is one of the reasons why trucks have diesel engines and not gasoline engines.
In the case of the gas engine, it results in the emission advantage becoming significantly smaller.
Dual-fuel engines have been developed in order to use natural gas as a fuel with the diesel combustion process. If you are interested in this concept, write me in the comments. It would be worth a separate article.
Due to their poorer efficiency, gasoline engines tend to have poorer efficiency and thus higher CO2 emissions than diesel engines.
Gas engines powered by CNG tend to have a CO2 advantage, despite their poorer efficiency.
How harmful to the climate is natural gas?
If natural gas is used, it must under no circumstances be released in the atmosphere unburned. Otherwise, the climate impact is devastating.
Unfortunately, this is not uncommon. I guess you might have read or heard about leaking gas pipelines or blow-offs during transportation.
If you are considering using gas engines, please be aware of this. In my opinion, this is underestimated, when people consider Natural Gas as a climate-friendly alternative.
Is biogas a sustainable fuel?
We know the generation of biogas from biomass, which takes place in the present and therefore does not use the fossil fuels of the early days.
Instead of natural gas, biogas can be used as fuel in vehicle engines.
When burning biogas, CO2 is generated just like when burning natural gas.
However, the production of the biogas is based on carbon, which is sourced from regrowing, sustainable materials. This means that the CO2 balance is neutral.
That would be a suitable way of reducing the greenhouse effect.
I see it rather pessimistically that we would be able to produce as much biogas as we need for the transport of all goods. (In Germany in 2020 28,6 Million tons of diesel have been used according to statista)
The plants that do photosynthesis for us need a lot of space and grow relatively slowly.
That was a lot of information.
You are probably interested in how CO2 emissions can be reduced in road transport. You can read about this in the many articles on how to reduce energy and fuel consumption. You can find them in the “Optimize consumption” category.
.If you have questions, don’t hesitate to write them in the comment. I will do my best to answer.



